X-Ray Diffraction Testing & Analysis (XRD)
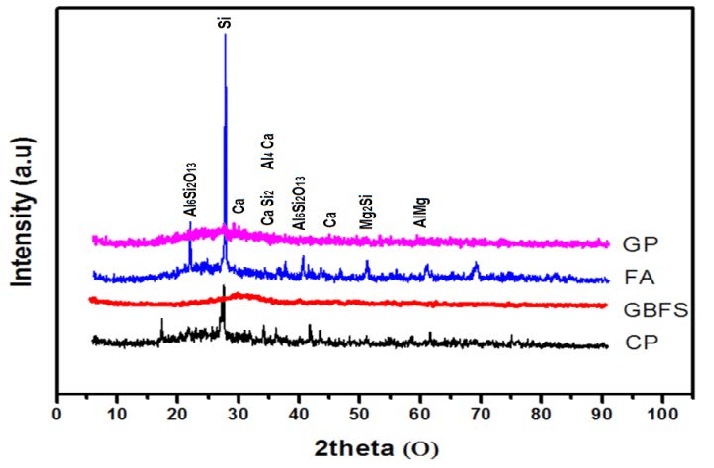
X Ray Diffraction Analysis is important to investigate crystalline material structure as well as atomic arrangement, crystallite size and imperfections. It is pivotal for the safety and efficacy of products. XRD is a valuable tool for analysis and testing of materials used in pharmaceuticals, food, cosmetics, studies in nanomaterials, blood, forensics, geological grading of minerals, electronics etc.
XRD Analysis in the Pharmaceutical Industry
In the pharmaceutical industry, XRD analysis plays an important role in the development of new drugs, as it helps to characterize active materials as well as tests material at different stages of manufacturing so that quality control is effectively maintained. All these procedures help to improve the formulation, analyze the compatibility of excipients, detect bioavailability and improve on factors of stability in different environments.
XRD Analysis in Food Industry
In the food industry, several analytical techniques are used to monitor food additives in the manufacturing process. XRD provides information on polymorphism (occurrence in several forms), a degree of crystallinity which influences hardness, density, transparency and diffusion of additives and amorphism (being available without crystallization even in the minute particles). These three parameters are tested in foods so as to control the texture and stability of foods under different storage conditions. XRD is particularly useful in conducting studies on food ingredients to develop quality products and especially tests starches, fats, chocolates or candies. Quality is particularly important for the development of foods so that they have the exact flavor, texture, and taste and should not be over sweet, salty, sour or bitter.
XRD Analysis in Cosmetics Industry
Cosmetics are composed of a number of nanoparticles to improve cosmetic features, especially in lotions, soaps, toothpaste, and conditioners. These cosmetics are popular but the compounds used in them can penetrate the skin and cause allergies and toxins. Therefore, XRD is used for testing nanoparticles for the safe development of cosmetics products so that their cosmetic features remain intact but products remain safe.
Drug Developement
• A pharmaceutical formulation is a complex mixture of several compounds which comprise of the
active drug ingredient together with other ingredients called excipients which act as fillers,
binders, lubricants, colorants, sweeteners, disintegrators, etc to achieve the desired properties
of a dosage form.
• X-ray diffraction or XRD has shown a huge potential in new drug development, manufacturing and
quality control of manufactured pharmaceutical formulations.
• The greatest asset of the XRD technique is its non-destructive nature and therefore the sample is
available for further analytical confirmations.
• Pharmaceutical formulations are available as tablets, capsules, syrups, injectables, creams, lotions,
aerosol sprays, etc X-ray diffraction provides valuable information on polymorphism, degree of
crystallinity and amorphous character of solid formulations. In other words, it is an ideal
fingerprinting technique for such formulations.
• The presence of a multitude of materials adds to the complexity of the formulation and a research
scientist has to try out various permutations and combinations before the formulation is approved for
commercialization.
• XRD is certainly helpful to the development chemist as it provides valuable details on the degree
of crystallinity and amorphous content of synthetic mixtures. Crystalline impurities present can be
quantified down to 0.05% levels.
• XRD data is accepted for new product registrations and patent applications. Single crystal
structure of the active ingredient and powder pattern of the finished formulation are essential
prerequisites for registration of new patents.
The development chemist has to collect important details on the following parameters before a product moves from laboratory to the manufacturing unit;
• Crystal structure– The lattice type and dimensions of a unit cell need to be specified for the crystalline content• Polymorphism– Polymorphic content can impact properties such as solubility and dissolution rate, bioavailability and stability so it is important to collect details on polymorphic properties of ingredients of a drug material.
• Percentage of crystallinity– The percent crystallinity is a valuable parameter for drug dosage form. It has significant influence on manufacturing and processing as well as pharmacological behaviour. XRD tests can effectively determine the percent crystallinity by measuring the volume concentration of an amorphous filler to a crystalline active matrix within the pharmaceutical product’s dosage form.
• Compatibility with excipients– XRD makes it an ideal choice for studies on active drug- excipient combinations. A detailed study of the chosen excipients with active pharmaceutical ingredient is a must for consistency of properties such as drug release and bio- availability.
• Crystallography- The general powder diffraction method can be used to successfully determine the crystal structure of a material, but usually, determining the dimensions and lattice type of the unit cell is sufficient for indexing.This method of analysis is especially important when the polymorphs of certain registered drugs whose patents are about to expire have to be characterized. X-ray diffraction analysis also allows identifying the atomic structure within the unit cell, preferred orientation, site occupancy, etc.
• Stability Studies-The stability of a pharmaceutical compound can be characterized along with the occurrence of hydration and dehydration processes by carrying out in situ powder diffraction studies as a function of temperature and relative humidity. It is possible to carry out these XRD methods at any phase of the drug development process.
• Dosage Uniformity-At our XRD testing labs, materials can be tested by subjecting them to conditions in which they will be actually used in specific applications. This is an important application of XRD analysis that helps to determine and monitor dosage uniformity.The process to determine that involves analyzing the actual percentages of each of the active ingredients in the final dosage form of the drug along with the percentage of other packing ingredients used in their amorphous or crystalline form.
Manufacturing Process Control
Manufacturing process can involve morphological changes in crystalline phase due to introduction of stress forces. Such changes can influence a drug’s bioavailability. XRD analysis is quite useful in the analysis and monitoring of the crystal morphology of the excipients used in drug formulation. Through the XRD method, any changes that may have occurred in the crystalline state of the excipients or the morphology of fillers in the final product can be detected. With the X’Celerator, the lower limit of detection has been significantly diminished for minority phases. The nondestructive nature of XRD analysis makes it an ideal choice to fix the safe tableting pressure range so that the dosage form achieves its targeted dissolution rate and bio availability. With its diverse range of applications XRD is gaining a high degree of popularity in pharmaceutical manufacturing and quality certifying laboratories.
 Admin Register
Admin Register
 Alcohol
Alcohol  Baby Care
Baby Care  Bakery, Cakes & Dairy
Bakery, Cakes & Dairy  Beauty-&-Hygiene
Beauty-&-Hygiene  Beverages
Beverages  Cleaning & Household
Cleaning & Household  Construction Materials
Construction Materials  Foodgrains, Oil & Masala
Foodgrains, Oil & Masala  Medicine
Medicine  Medicine Related Cosmetics
Medicine Related Cosmetics  Non-Veg
Non-Veg  Pesticides
Pesticides  Snacks & Branded Foods
Snacks & Branded Foods  Supplement
Supplement  Veterinary
Veterinary 